That is where a second unique formulation
The "chemical coolant" comes into action.
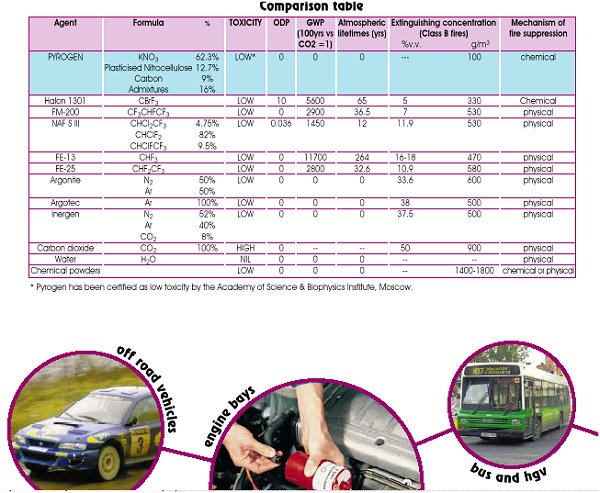
The secret to Pyrogen's' power is in two unique formulations contained in Pyrogen canister - the solid aerosol-generating chemical and the solid chemical coolant.
The solid aerosol-generating chemical is a thermoplastic mixture consisting of an oxidizer, a combustible binder and technological additives. The oxidizer is a solid potassium nitrate (KNO3(s)), the combustible binder is a solid plasticized nitro-cellulose (CnHmNpOq(s)) and technological additives include carbon (C(s)) as an activator of the oxidizer's decomposition, chemical and mechanical stabilizers and some other ingredients.
When ignited the solid-generating chemical undergoes a combustion reaction, which can schematically be represented as follows:
KNO3 (s) + CnHmNpOq (s) + C(s) = KHCO3 (s) + K2CO3 (s) + CO2 (g)+ N2(g) + H2O (g)
Combustion products consist of potassium carbonates (KHCO3 ,K2CO3), carbon dioxide gas (CO2 (g)), nitrogen gas ( N2 (g) ) and water vapor (H2O(g)) and represent the actual extinguishing agent.
As the reaction temperatures are high, potassium carbonates are formed in the gas phase, but as the vapor cools, the potassium carbonates condense to a liquid and then a solid. As solid potassium carbonates are produced by condensation, the particle size is very small - approximately 1 micron. Micron sized solid particles mix with the gaseous carbon dioxide, nitrogen and water into a uniform homogeneous gas - like phase - an aerosol.
Thus, Pyrogen extinguishing aerosol is a suspension of the micron sized solid particles, mainly potassium carbonates, in the gas mix of carbon dioxide, nitrogen and water vapor. Being a combustion product of the aerosol-generating chemical, Pyrogen aerosol is hot upon formation. Although, Pyrogen aerosol is the most effective in terms of the actual fire extinguishment when in its hottest state, the negative impacts of very high temperatures are obvious. Ejection of flame at the discharge outlet and poor distribution within a protected enclosure is the main of those impacts and should be eliminated.

When the hot Pyrogen aerosol passes through the coolant, the coolant decomposes absorbing a great amount of heat.
Pyrogen chemical coolant is a polymer composition highly impregnated with endothermic ingredients - substances that decompose at 200-300 ° C without melting, generate gases and absorb approximately 400 Cal of heat per one kilogram of their mass. Cooling is effected at a fantastic rate of 400 degrees per second.
Application of the Pyrogen coolant enables the arrest flames at the discharge outlet and provides uniform distribution of the aerosol within the area, which certainly contributes to the reliability and safety of the extinguishment. Moreover, additional amounts of inert gases are formed due to a thermal decomposition of the coolant, which contribute to the effectiveness of the extinguishment.